
Equation of State
What is an equation of state ? What are the most common equations of state ?
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
| Section summary |
|---|
| 1. Definition of an
equation of state |
| 2. Equations of
state |
| 3. Use of equations of state |
1. Definition of an equation of state
What is an equation of state ?
An equation of state represents the behavior of real fluids by linking mathematically pressure, volume, temperature and molar quantity
Equation of state = f(P,V,T,n)
With
P = absolute pressure of the gas
V = volume of the gas
n = quantity of the gas (in moles)
T = absolute temperature of the gas
From the definition above, the simplest equation of state is the ideal gas law
(only for gas) and the real
fluid law (with the compressibility factor). They are however
very simple and are commonly not referred to as equation of state.
The 1st equation of state as such is the Van der Waals equation.
Other equations were later proposed.
2. Equation of states
What are the most common equations of state ?
2.1 Van der Waals equation of state

With
a = constant depending on the gas, the ratio a/V2 is
called the bonding pressure. It described that real gases have a
pressure less than the ideal gas modelization
(Preal_gas = Pideal_gas - a/V2)
b = constant depending on the gas and is called co-volume. It
represents the fact that real molecules take more space than ideal
ones, creating a bigger volume of gas
(Vreal_gas = Videal_gas + b)
3.2 Redlich-Kwong equation of state

The parameters a and b can be calculated thanks to the critical conditions of the substance.
To be noted the alternative expression of the Redlich-Kwong equation that can be obtained by changing the variables :

A and B can then be expressed as a function of the reduced temperature, reduced pressure and critical pressure which allows to perform calculations easier.
To be noted that the equation of state also applies to gas mixtures by summing the A and B parameters of each constituants ponderated by the molar fraction of the component in the mixture (Am = sum of yi.Ai and Bm = sum of yi.Bi).
3.3 Soave-Redlich-Kong equation of state

3.4 Peng-Robinson equation of state
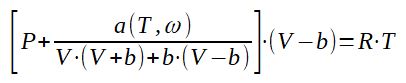
3. Use of equations of state
What can be calculated with the equation of states ?
The following fluids properties can be calculated thanks to an equation of state :
- Volume of the fluid
- Density of the fluid
- Vapor pressure
- Liquid vapor equilibria
- Enthalpy of the fluid
- Entropy of the fluid
Note : liquid vapor equilibria are calculated thanks to fugacity coefficients. Enthalpy and entropy are calculated by correcting the value obtained for an ideal gas
Only few equations of states among all that have been proposed are mentioned in this page. It is difficult to define the "best" equation of state. It is more accurate to say that each equation of state is working better than other in specific conditions, up to the engineer to chose the equation of state better adapted to the conditions he wishes to represent.