Oxygen saturation concentration of water calculation step by step
How much oxygen can be dissolved in water at equilibrium ?
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. STEP 1 : Gather data
2. STEP 2 : Calculate the partial pressure of oxygen
3. STEP 3 : Calculate the mole fraction of oxygen in water
5. STEP 5 : Calculate the saturation concentration of oxygen in water in mg / l
Calculating the amount of oxygen dissolved in water can be useful in many Engineering disciplines but it is especially the case in Environmental Engineering. This page is presenting a step by step method to calculate the saturation concentration of oxygen in water. The calculations are focusing on equilibrium of water with air but a similar method could be used with another gas mixture containing oxygen, or even to calculate the saturation concentration in water of another component of the gas.
1. STEP 1 : Gather data
In order to calculate the saturation concentration of oxygen in water, the Engineer must know the following data :
pg = partial pressure of oxygen in the gas (mol O2
/ mol gas)
PT = total gas pressure (atm)
T = temperature of the water (c)
Henry's law constant for oxygen at the temperature of interest
2. STEP 2 : Calculate the partial pressure of oxygen
The partial pressure of oxygen can be calculated as Pg = PO2/PT. Air is made of around 20.95% of oxygen, which means that the partial pressure of oxygen in air is about 0.2095
3. STEP 3 : Calculate the mole fraction of oxygen in water
The calculation of the mole concentration of oxygen per mole of water is done thanks to Henry's law which links the mole fraction of a component in the liquid phase to the partial pressure of this component in the gas phase in equilibrium. It is also required to use Henry's constant for this component.
xg = PT/H * Pg
With :
xg = molar fraction of oxygen in the water (mol O2 / mol water)
PT = total pressure of the gas (atm)
H = Henry's constant (atm)
Pg = partial pressure of oxygen in the gas phase (-)
4. STEP 4 : Calculate the concentration of oxygen per liter of
water
The next step is to calculate the concentration of oxygen in mole per liter of water. For this it is necessary to come back to the definition of the molar fraction of oxygen in water :
xg = ng / (ng + nw)
With :
xg = molar fraction of oxygen in the water (mol O2 / mol water)
ng = number of mole of oxygen in one liter of water (mol)
nw = number of mole water in one liter of water (mol)
To simplify the calculations it can be assumed that nw >> ng which means that :
xg = ng / nw
ng = xg * nw
5. STEP 5 : Calculate the saturation concentration of oxygen in
water in mg / l
Now that the molar concentration per liter of water is known, it can be converted to weight concentration by multiplying by the molecular weight of O2.
Cg = ng * MgWith :
Cg = mass concentration of oxygen in water (g/l water)
ng = number of mole of oxygen in one liter of water (mol)
Mg = molecular weight of oxygen (g/mol)
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
6. Step by Step example : calculation of oxygen saturation
concentration in water
An Engineer would like to calculate the amount of oxygen that should be dissolved in a pond at equilibrium (he can then compare to the actual amount measured by analysis to diagnose possible water quality issues)
Step 1 : collect the data
PT = total gas pressure (atm) = 1 atm
T = temperature of the water (c) = 20 c
Henry's law constant for oxygen at the temperature of interest =
41100 atm for oxygen in water
Step 2 : Calculate the partial pressure of oxygen
Pg = PO2/PT = 0.2095
Step 3 : Calculate the mole fraction of oxygen in water
xg = PT/H * Pg = 1 (atm) / 41100 (atm (mol gas / mol air) / (mol gas / mole water) * 0.2095 (mol gas / mol air) = 5.097 * 10-6 (mol gas / mole water)
Step 4 : Calculate the concentration of oxygen per liter of water
nw = 1000 / 18 = 55.56 mol of water / l of water
ng = xg * nw = 5.097 * 10-6 * 55.56 = 2.83 * 10-4 mol gas / liter of water
Step 5 : Calculate the saturation concentration of oxygen in water in mg / l
Cg = 2.83 * 10-4 * 32 * 1000 = 9.06 mg/l
The saturation concentration of oxygen in water at 20c is 9.06 mg/l
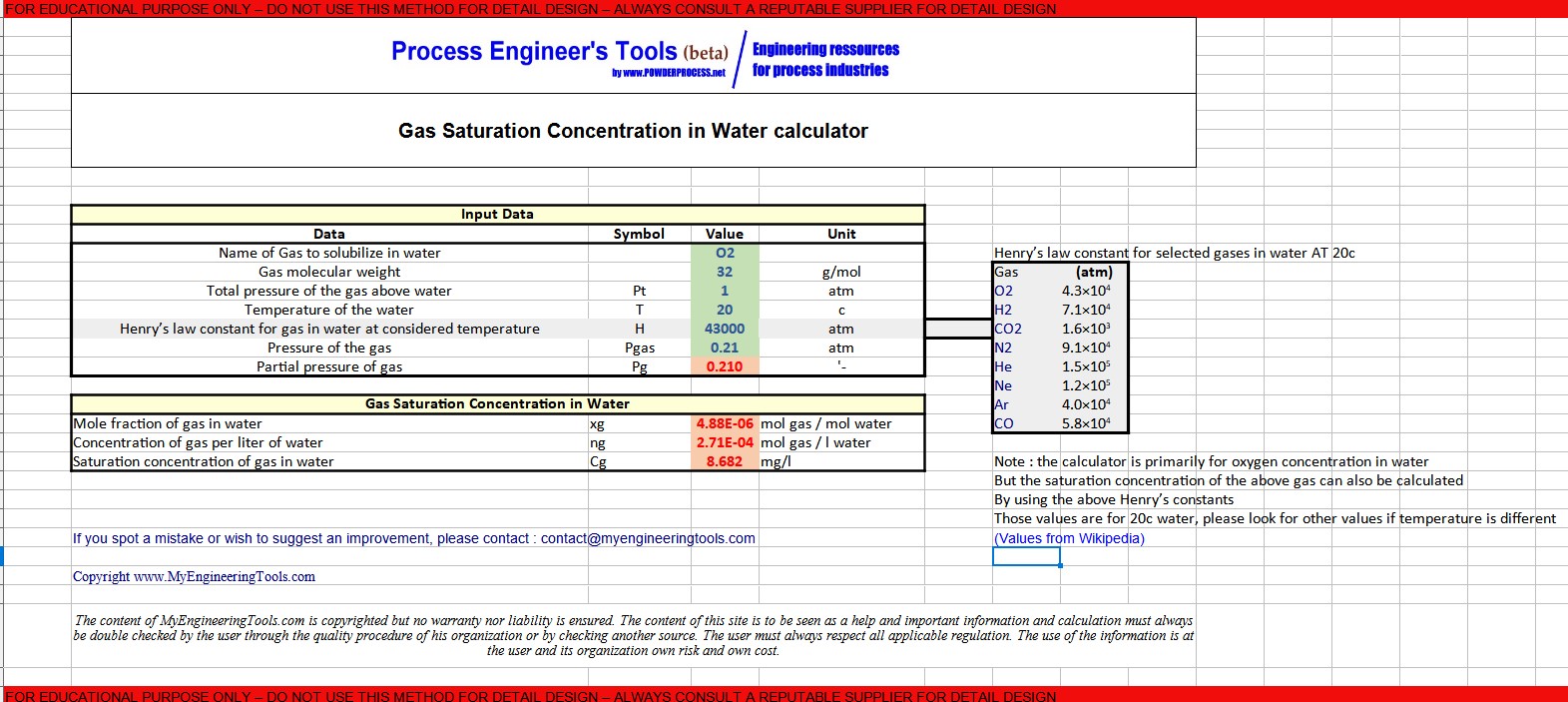
7. Gas saturation concentration in water calculator Excel
MyEngineeringTools.com has developed a free Excel calculator that allows you to calculate the gas saturation concentration in water : Gas saturation concentration in Water calculator Excel
Warning : this calculator is provided to illustrate the concepts mentioned in this webpage, it is not intended for detail design. It is not a commercial product, no guarantee is given on the results. Please consult a reputable designer for all detail design you may need.
Sources
[Chopey] Handbook of Chemical Engineering calculations, Chopey et al, McGraw Hill, 2004