
Viscosity of common gases
Viscosity of gases at atmospheric pressure from -150 to 600 celsius, among which air, nitrogen, oxygen, steam
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. Variation of gas viscosity with temperature
1. Variation of gas viscosity with temperature
How does viscosity of gases vary with temperature ?
The viscosity of gases is, in general, increasing with temperature. The additional movement of the gas molecules is actually causing more contact, thus interaction in between molecules leading to an increase in viscosity.
This page is detailing the viscosity in between -150°c and +600°c for common gases : Oxygen, Air, Nitrogen, Carbon Dioxide, Steam, Hydrogen.2. Graph of viscosity changes with temperature for common gases
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
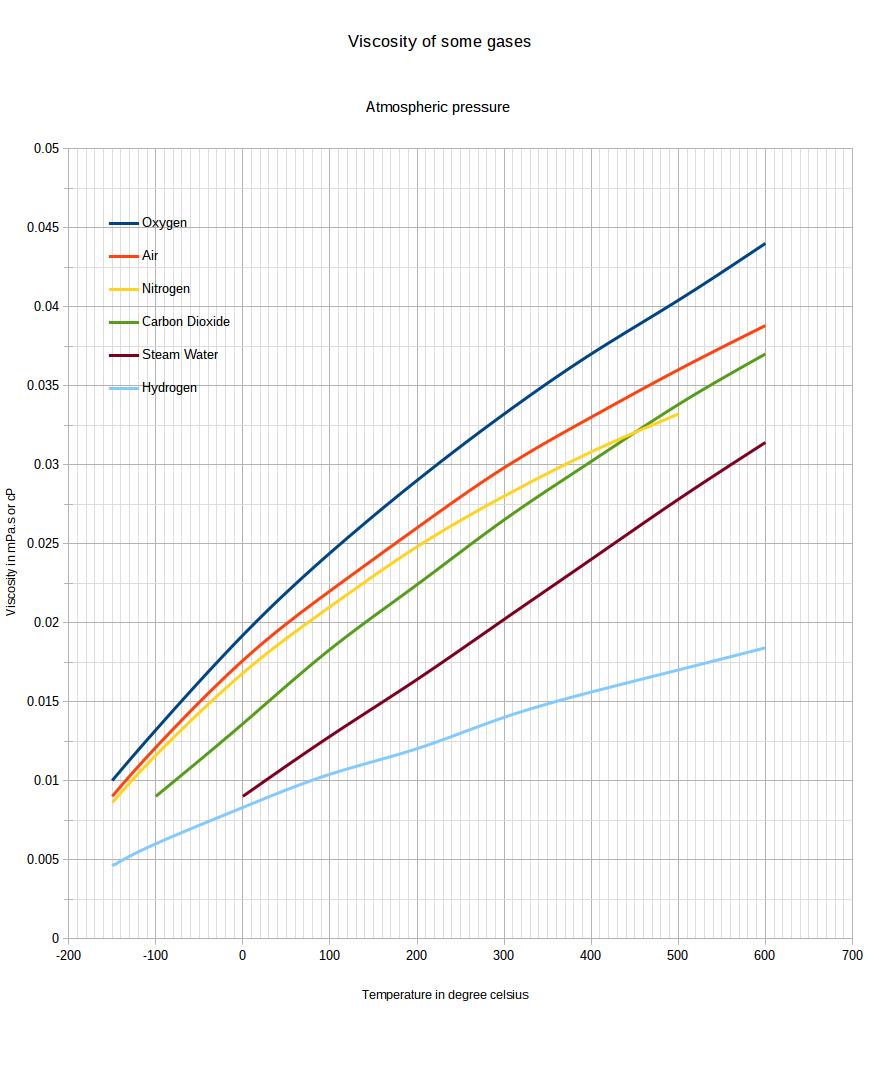
3. Data table : variation of gases viscosity as a function of temperature
| Viscosity in mPa.s or cP | ||||||
| Temperature in celsius | Oxygen | Air | Nitrogen | Carbon Dioxide | Steam Water | Hydrogen |
| -150 | 0.01 | 0.009 | 0.0086 | 0.0046 | ||
| -100 | 0.0132 | 0.0121 | 0.0116 | 0.009 | 0.006 | |
| 0 | 0.0192 | 0.0176 | 0.0168 | 0.0136 | 0.009 | 0.0083 |
| 100 | 0.0244 | 0.022 | 0.021 | 0.0183 | 0.0128 | 0.0104 |
| 200 | 0.029 | 0.026 | 0.0248 | 0.0224 | 0.0164 | 0.01202 |
| 300 | 0.0332 | 0.0298 | 0.028 | 0.0265 | 0.0202 | 0.014 |
| 400 | 0.037 | 0.033 | 0.0308 | 0.0302 | 0.024 | 0.0156 |
| 500 | 0.0404 | 0.036 | 0.0332 | 0.0338 | 0.0278 | 0.017 |
| 600 | 0.044 | 0.0388 | 0.037 | 0.0314 | 0.0184 | |
Note : Approximate values
The data table below is aggregated from different sources (warning : not verified individually), it gives the viscosity of 30+ common gases as a function of the temperature.
| Viscosity in μPa.s | |||||||||
| 100K | 200K | 273K | 300K | 400K | 500K | 600K | |||
| C₂H₂ | Acethylene | Viscosity of the gas Acethylene | 9.35 | ||||||
| C2H2 | Acetylene | Viscosity of the gas Acetylene | 10.4 | 13.5 | 16.5 | ||||
| Air | Air | Viscosity of the gas Air | 7.1 | 13.3 | 18.5 | 23.1 | 27.1 | 30.8 | |
| Air | Air | Viscosity of the gas Air | 17.3 | ||||||
| H3N | Ammonia | Viscosity of the gas Ammonia | 10.2 | 14 | 17.9 | 21.7 | |||
| NH₃ | Ammonia | Viscosity of the gas Ammonia | 9.18 | ||||||
| Ar | Argon | Viscosity of the gas Argon | 20.9 | ||||||
| Ar | Argon (P=0) | Viscosity of the gas Argon (P=0) | 8.1 | 15.9 | 22.7 | 28.6 | 33.9 | 38.8 | |
| BF3 | Borontrifluoride | Viscosity of the gas Borontrifluoride | 12.3 | 17.1 | 21.7 | 26.1 | 30.2 | ||
| C4H10 | Butane | Viscosity of the gas Butane | 7.5 | 9.9 | 12.2 | 14.5 | |||
| C₄H₁₀ | Butane | Viscosity of the gas Butane | 8.1 | ||||||
| CO₂ | Carbon Dioxide | Viscosity of the gas Carbon Dioxide | 13.7 | ||||||
| CO | Carbon Monoxide | Viscosity of the gas Carbon Monoxide | 16.6 | ||||||
| CO2 | Carbondioxide | Viscosity of the gas Carbondioxide | 10.1 | 15 | 19.7 | 24 | 28 | ||
| CO | Carbonmonoxide | Viscosity of the gas Carbonmonoxide | 6.7 | 12.9 | 17.8 | 22.1 | 25.8 | 29.1 | |
| Cl₂ | Chlor | Viscosity of the gas Chlor | 12 | ||||||
| CHCl3 | Chloroform | Viscosity of the gas Chloroform | 10.2 | 13.7 | 16.9 | 20.1 | |||
| D2 | Deuterium(P=0) | Viscosity of the gas Deuterium(P=0) | 5.9 | 9.6 | 12.6 | 15.4 | 17.9 | 20.3 | |
| D2O | Deuteriumoxide(P=0) | Viscosity of the gas Deuteriumoxide(P=0) | 10.2 | 13.7 | 17.8 | 22 | |||
| C4H10O | Diethylether | Viscosity of the gas Diethylether | 7.6 | 10.1 | 12.4 | ||||
| C2H6 | Ethane | Viscosity of the gas Ethane | 6.4 | 9.4 | 12.2 | 14.8 | 17.1 | ||
| C₂H₆ | Ethane | Viscosity of the gas Ethane | 8.5 | ||||||
| C2H6O | Ethanol | Viscosity of the gas Ethanol | 11.6 | 14.5 | 17 | ||||
| C₂H₅Cl | Ethyl Chloride | Viscosity of the gas Ethyl Chloride | 9.4 | ||||||
| C2H4 | Ethylene | Viscosity of the gas Ethylene | 7 | 10.4 | 13.6 | 16.5 | 19.2 | ||
| C₂H₄ | Ethylene | Viscosity of the gas Ethylene | 9.85 | ||||||
| He | Helium | Viscosity of the gas Helium | 18.8 | ||||||
| He | Helium(P=0) | Viscosity of the gas Helium(P=0) | 9.6 | 15.1 | 19.9 | 24.3 | 28.3 | 32.2 | |
| C6H14 | Hexane | Viscosity of the gas Hexane | 8.6 | 10.8 | 12.8 | ||||
| H₂ | Hydrogen | Viscosity of the gas Hydrogen | 8.42 | ||||||
| HCl | Hydrogen Chloride | Viscosity of the gas Hydrogen Chloride | 146.4 | ||||||
| ClH | Hydrogenchloride | Viscosity of the gas Hydrogenchloride | 14.6 | 19.7 | 24.3 | ||||
| H2S | Hydrogensulfide | Viscosity of the gas Hydrogensulfide | 12.5 | 16.9 | 21.2 | 25.4 | |||
| C₄H₁₀ | i-Butane | Viscosity of the gas i-Butane | 7.47 | ||||||
| C4H10 | Isobutane | Viscosity of the gas Isobutane | 7.5 | 9.9 | 12.2 | 14.4 | |||
| Kr | Krypton | Viscosity of the gas Krypton | 23.2 | ||||||
| Kr | Krypton(P=0) | Viscosity of the gas Krypton(P=0) | 17.4 | 25.5 | 32.9 | 39.6 | 45.8 | ||
| CH₄ | Methane | Viscosity of the gas Methane | 10.3 | ||||||
| CH4 | Methane(P=0) | Viscosity of the gas Methane(P=0) | 3.9 | 7.7 | 11.1 | 14.2 | 17 | 19.5 | |
| CH4O | Methanol(P=0) | Viscosity of the gas Methanol(P=0) | 6.6 | 9.7 | 13 | 16.4 | 19.8 | ||
| CH₃Cl | Methyl Chloride | Viscosity of the gas Methyl Chloride | 9.89 | ||||||
| Ne | Neon | Viscosity of the gas Neon | 29.7 | ||||||
| Ne | Neon(P=0) | Viscosity of the gas Neon(P=0) | 14.4 | 24.1 | 31.9 | 38.6 | 44.8 | 50.6 | |
| NO | Nitricoxide | Viscosity of the gas Nitricoxide | 13.8 | 19.2 | 23.8 | 28 | 31.9 | ||
| N2 | Nitrogen | Viscosity of the gas Nitrogen | 7 | 12.9 | 17.9 | 22.2 | 26.1 | 29.6 | |
| N₂ | Nitrogen | Viscosity of the gas Nitrogen | 17 | ||||||
| NO | Nitrogen Oxide | Viscosity of the gas Nitrogen Oxide | 17.8 | ||||||
| N2O | Nitrousoxide(P=0) | Viscosity of the gas Nitrousoxide(P=0) | 10 | 15 | 19.8 | 24.1 | 27.9 | ||
| H2 | Normalhydrogen(P=0) | Viscosity of the gas Normalhydrogen(P=0) | 4.1 | 6.8 | 8.9 | 10.9 | 12.8 | 14.5 | |
| O2 | Oxygen | Viscosity of the gas Oxygen | 7.7 | 14.7 | 20.7 | 25.8 | 30.5 | 34.7 | |
| O₂ | Oxygen | Viscosity of the gas Oxygen | 20.3 | ||||||
| C5H12 | Pentane | Viscosity of the gas Pentane | 6.7 | 9.2 | 11.4 | 13.4 | |||
| C₅H1₂ | Pentane | Viscosity of the gas Pentane | 8.74 | ||||||
| C3H8 | Propane | Viscosity of the gas Propane | 8.2 | 10.8 | 13.3 | 15.6 | |||
| C₃H₈ | Propane | Viscosity of the gas Propane | 7.95 | ||||||
| C₃H₆ | Propene | Viscosity of the gas Propene | 8.35 | ||||||
| O2S | Sulfurdioxide | Viscosity of the gas Sulfurdioxide | 8.6 | 12.9 | 17.5 | 21.7 | |||
| F6S | Sulfurhexafluoride(P=0) | Viscosity of the gas Sulfurhexafluoride(P=0) | 15.3 | 19.7 | 23.8 | 27.6 | |||
| SO₂ | Sulphur Dioxide | Viscosity of the gas Sulphur Dioxide | 11.7 | ||||||
| H₂S | Sulphur Hydrogen | Viscosity of the gas Sulphur Hydrogen | 11.66 | ||||||
| H2O | Water(P=0) | Viscosity of the gas Water(P=0) | 9.8 | 13.4 | 17.3 | 21.4 | |||
| Xe | Xenon | Viscosity of the gas Xenon | 21 | ||||||
| Xe | Xenon(P=0) | Viscosity of the gas Xenon(P=0) | 15.7 | 23.2 | 30.5 | 37.2 | 43.5 | ||
4. Variation with pressure
How does viscosity of gases vary with pressure ?
If the pressure increases, gases viscosity is usually increasing as well.