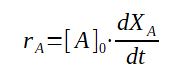Batch Reactors perfectly stirred : reaction conversion
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. Reaction conversion
2. Batch reactor : reaction speed as a function of conversion
3. Conversion in case of multiple reactions
It can be interesting to define a conversion rate in order to express the reaction speed. The conversion is based on the limited reactant in a reaction.
1. Reaction conversion
What is the conversion in a chemical reaction ?
The reaction conversion is a measure of the progress of the reaction referring to the limiting reactant. The reaction will indeed not be able to go further once one of the reactant is consumed. The conversion rate of the reaction, based on the limiting reactant (named A hereafter), can then be defined a XA by the following equation :
nA = nA,0 * (1 + νA*XA)
With
nA = quantity of the limiting reactant A at time t (mol)
nA,0 = quantity of the limiting reactant A at t=0 (mol)
νA = stoechiometric coefficient associated to the
limiting reactant A in the reaction considered. As we refer to a
reactant, νA < 0
XA = conversion rate relatively to the limiting
reactant A
At t=0 : XA = 0
At t = end of reaction : XA = -1/νA
2. Batch reactor : reaction speed as a function of conversion
The mass balance in a batch reactor, perfectly stirred and isotherm allows to show that the reaction speed of a reactant A is :
rA = 1/(V*νA) * dnA/dt
For the limiting reactant A, the quantity of material at an instant t is :
nA = nA,0 * (1 + νA*XA)
Thus the reaction rate can be expressed as a function of the conversion :
rA = nA,0/V * dXA/dt
When the reactor has a constant volume, we can use concentration (mol/s) instead of quantity of material (mol), which is very often more practical, the reaction speed as function of the conversion becomes then :
rA = [A]0 * dXA/dt
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
3. Conversion in case of multiple reactions
When the limiting reactant is involved in multiple reactions, the relations above can be generalized the following way :

If the reactor is at constant volume, we also have :